10 Things We Learned About OTC Hearing Aids in the Last Year
By Kate Carr, President Hearing Industries Association (HIA) and Thomas A. Powers, PhD, Strategic Advisor, HIA
November 8, 2023
 It has been just over one year since the United States Food and Drug Administration (FDA) made hearing aids an over-the-counter (OTC) product. For those in the hearing care profession and the hearing industry, the wait for the final regulation1 of OTC hearing aids was long, somewhat stressful, and uncertain. How would persons with hearing difficulty react? Would OTC diminish the businesses of hearing professionals? Would it be the revolution some expected for hearing health? Would the rule address and accomplish the intent of the original legislation2 passed in 2017? This article provides a review of the goals of the OTC hearing aid rule and what we have observed in the market since.
It has been just over one year since the United States Food and Drug Administration (FDA) made hearing aids an over-the-counter (OTC) product. For those in the hearing care profession and the hearing industry, the wait for the final regulation1 of OTC hearing aids was long, somewhat stressful, and uncertain. How would persons with hearing difficulty react? Would OTC diminish the businesses of hearing professionals? Would it be the revolution some expected for hearing health? Would the rule address and accomplish the intent of the original legislation2 passed in 2017? This article provides a review of the goals of the OTC hearing aid rule and what we have observed in the market since.
The goals behind the OTC hearing aid regulation were straightforward: accessibility, affordability and innovation.
Now, more than one year after the effective date of the OTC hearing aid rule promulgated by FDA, we have a few observations to share.
- Customer. The OTC customer is younger; wants a simple process without an appointment or prescription; is more of a situational user for meetings and social engagements; and inclined to purchase from a brand they recognize.
- Market. Accessibility and competition are certainly there. More than 40 companies are selling OTC devices with over 80 companies filing registrations or 510(k) applications with the FDA. A number of these devices were previously available as direct to consumer products and now can be obtained at retail stores, pharmacies, online and even from some hearing care professionals.
- Branding and Partnerships. Some companies have recognized the power of a known brand. Some examples include the partnership between Nuheara and HP, WSAudiology and Sony, GN’s branding with Jabra, and Sonova’s acquisition of the audio division of Sennheiser. We note a fact from Hearing Industries Association's (HIA) MarkeTrak 223 study that revealed that 71% of hearing aid users did not know the brand of the hearing aid which may have led to the collaboration with the consumer brands mentioned above.
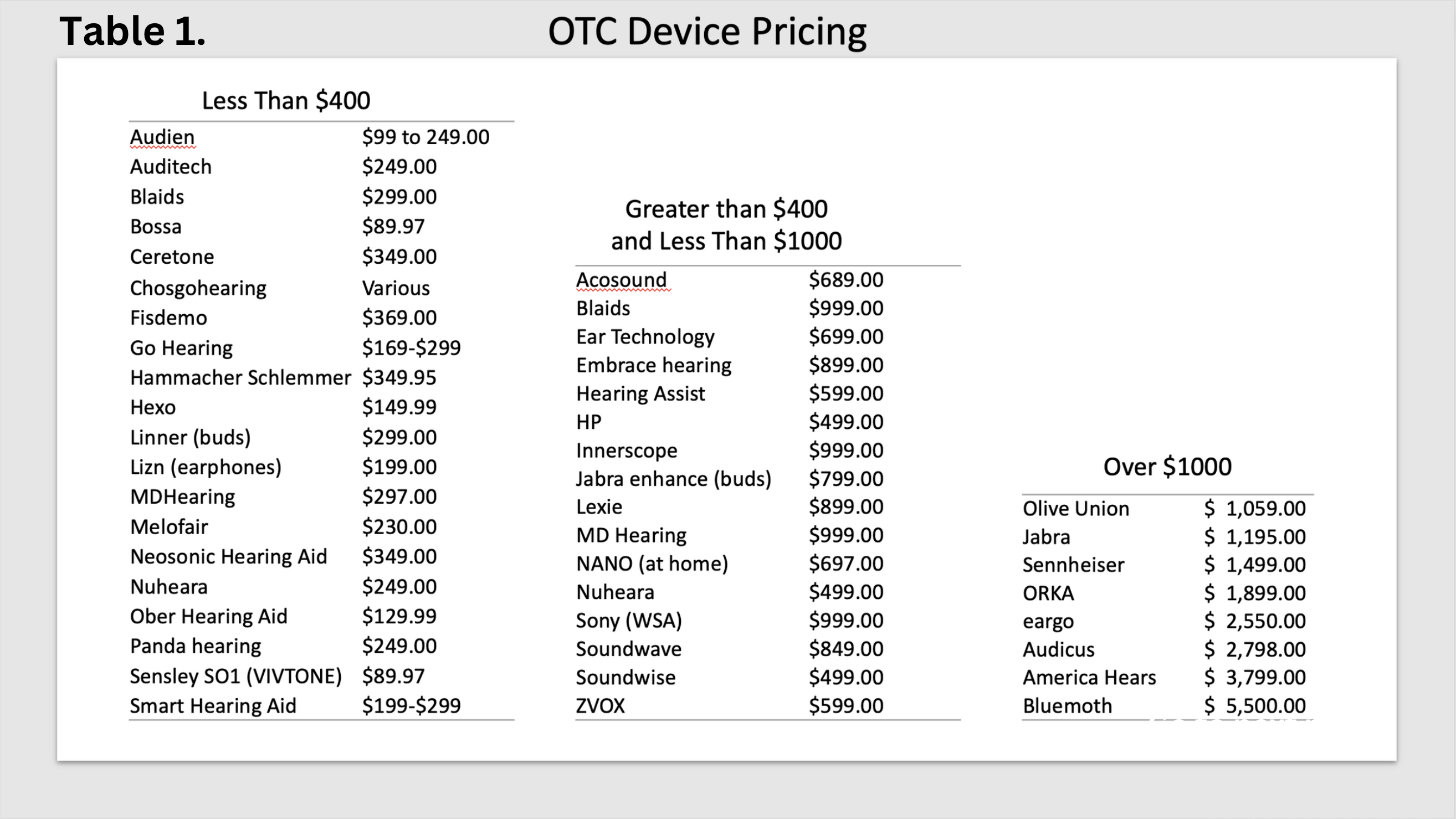 Cost. Affordability is not an issue with prices ranging from $89.97 to more than $2,000. That is not to say that an OTC buyer understands the technology differences between a lower cost hearing aid and a premium device. Secondly, they may not understand the potential benefits, or lack of, provided by many of the devices at the lower end of the price spectrum. Table 1 illustrates the various price points that we found in an internet search.
Cost. Affordability is not an issue with prices ranging from $89.97 to more than $2,000. That is not to say that an OTC buyer understands the technology differences between a lower cost hearing aid and a premium device. Secondly, they may not understand the potential benefits, or lack of, provided by many of the devices at the lower end of the price spectrum. Table 1 illustrates the various price points that we found in an internet search. - Insurance. Currently, it appears that insurance coverage for OTC hearing aids is very limited. Given how new the market is, insurance companies will need to adapt their plans. Over half of Medicare beneficiaries are currently enrolled in Medicare Advantage (MA), and almost all MA plans offer some type of hearing benefit. MA plans continue to innovate, and we are closely tracking developments and changes. Will coverage shift from prescription hearing aids to OTC? Our crystal ball is fuzzy on that question.
- Innovation. When we look at innovation, we are not seeing major new developments at this time except for some new form factors that are available or will soon come to market. Both prescription and OTC hearing aids share many of the same technological features including directional microphones; feedback reduction; noise suppression; rechargeable batteries; and wireless connectivity.
- Self-Fit Availability. The FDA regulations provided for OTC devices with pre-set listening environments such as quiet, TV and noisy environments. It also provided a second category for Self-Fitting OTC devices, which the consumer can adjust using an app or online resources. The FDA requires self-fitting OTC devices to obtain clearance through the FDA 510(k) process before the device can be sold. This step takes time and resources and, so far, there are limited self-fitted devices available. It’s worth noting that the cost of these devices tends to be higher.
- Bad actors. One of our major concerns is the proliferation of misleading advertising, unsubstantiated claims and companies not following the guidelines in the FDA OTC regulation. Examples include claims of restoring natural hearing, treatment of severe hearing loss, and our favorite fallacy – invisible hearing aids that use CIA technology. These examples, and more, have been raised with FDA to attempt to stem the rising tide of consumer confusion. We continue to track these claims.
- OTC sales and returns. Does anyone know how many OTC devices are being sold? Not really! While HIA tracks hearing aid sales for our members and provides a broad perspective of the market, no one is currently tracking OTC sales or returns. Some public information exists and reveals sales with returns of more than 30% for OTC devices. And we have heard that OTC buyers that receive some assistance from a hearing care professional or from customer service representations may have higher rates of satisfaction and lower returns to the company than those buyers who receive no assistance at all.
- Our best advice. If you are concerned about your hearing difficulty, our advice is to schedule an appointment for a hearing test with a hearing care professional. You will learn the extent of your hearing loss and be able to explore whether an OTC or prescription device would best suit your individual needs. For more information on hearing health, visit www.betterhearing.org
Will OTC succeed? There are those who believe that the market will evolve over time. And there are some who are much more skeptical. Our conclusion – time will tell!
 It has been just over one year since the United States Food and Drug Administration (FDA) made hearing aids an over-the-counter (OTC) product. For those in the hearing care profession and the hearing industry, the wait for the final regulation1 of OTC hearing aids was long, somewhat stressful, and uncertain. How would persons with hearing difficulty react? Would OTC diminish the businesses of hearing professionals? Would it be the revolution some expected for hearing health? Would the rule address and accomplish the intent of the original legislation2 passed in 2017? This article provides a review of the goals of the OTC hearing aid rule and what we have observed in the market since.
It has been just over one year since the United States Food and Drug Administration (FDA) made hearing aids an over-the-counter (OTC) product. For those in the hearing care profession and the hearing industry, the wait for the final regulation1 of OTC hearing aids was long, somewhat stressful, and uncertain. How would persons with hearing difficulty react? Would OTC diminish the businesses of hearing professionals? Would it be the revolution some expected for hearing health? Would the rule address and accomplish the intent of the original legislation2 passed in 2017? This article provides a review of the goals of the OTC hearing aid rule and what we have observed in the market since. Cost. Affordability is not an issue with prices ranging from $89.97 to more than $2,000. That is not to say that an OTC buyer understands the technology differences between a lower cost hearing aid and a premium device. Secondly, they may not understand the potential benefits, or lack of, provided by many of the devices at the lower end of the price spectrum. Table 1 illustrates the various price points that we found in an internet search.
Cost. Affordability is not an issue with prices ranging from $89.97 to more than $2,000. That is not to say that an OTC buyer understands the technology differences between a lower cost hearing aid and a premium device. Secondly, they may not understand the potential benefits, or lack of, provided by many of the devices at the lower end of the price spectrum. Table 1 illustrates the various price points that we found in an internet search.